Already widely present in the therapeutic arsenal, monoclonal antibodies still hold enormous potential for future decades: to remain competitive in the pharmaceutical industry, France must invest more widely in this research field. There is also the added alarming economic finding, that monoclonal antibodies only represent a cost item for the national health system. France is one of the main world consumers without the socio-economic benefits.
In an extremely competitive international context, lifting France to the top requires a strong academic community involvement on unresolved scientific issues, particularly for improved understanding of the pharmacology of monoclonal antibodies in humans.
The purpose is to improve their use, improve their design and development and to develop the best.
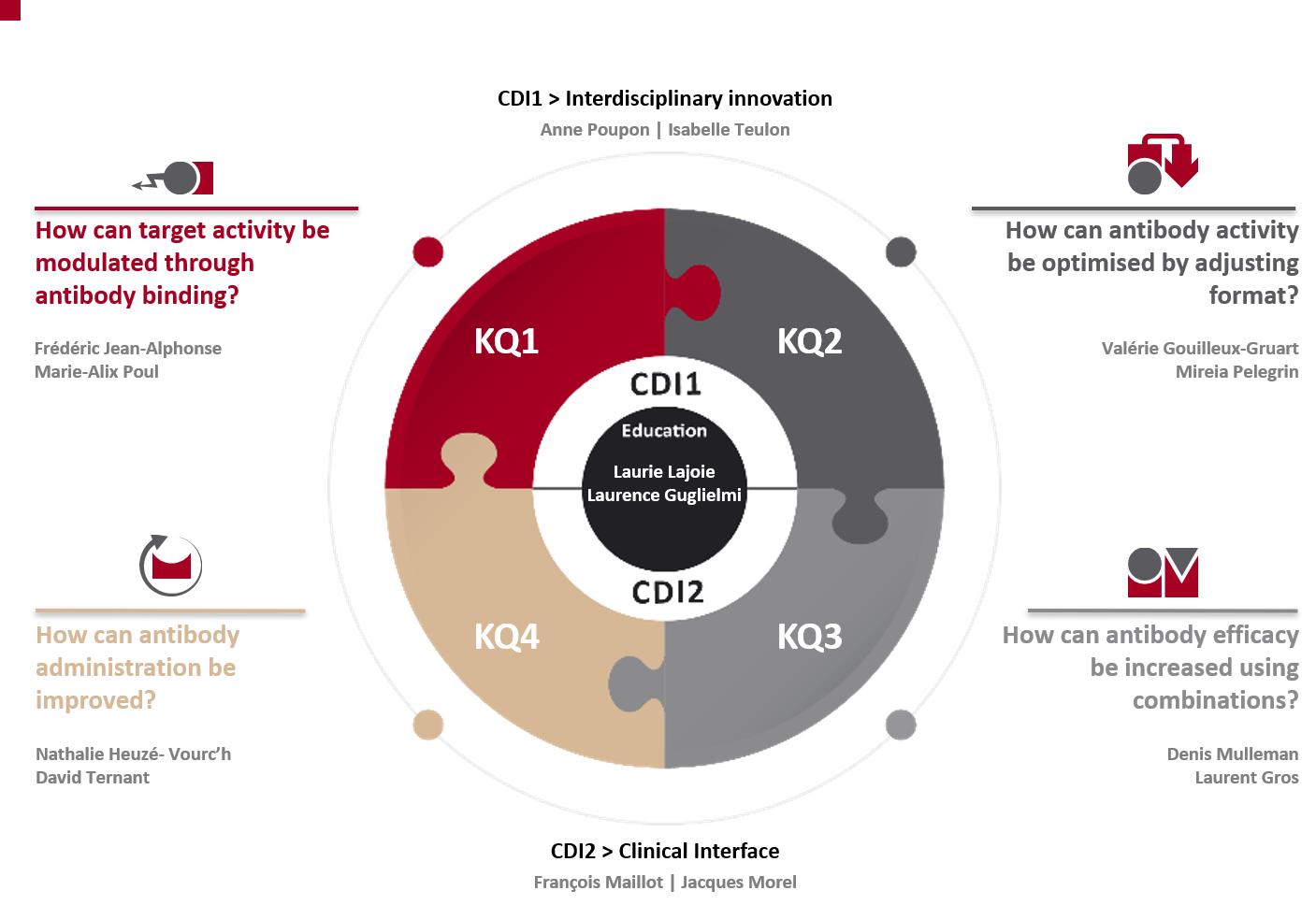
In response, MAbImprove partners have initially organized five work packages (WP). We have moved into four key questions (KQ) because it was difficult for potential partners to understand. “Key questions” appears as a better way to explain our lines of investigation going forward. Two central development instruments (CDI) were added as part of our recently renewed programme to provide all our researchers with transversal tools.
- KQ1- How can target activity be modulated through antibody binding?
- KQ2 – How can antibody activity be optimised by adjusting format?
- KQ3 – How can antibody efficacy be increased using combinations?
- KQ4 – How can antibody administration be improved?
- CDI1 – Interdisciplinary innovation
- CDI2 – Bio-clinical interface
Flagship projects
In addition, in order to boost & strengthen cooperation between teams, dedicated funding has been allocated to flagship projects that aim at bringing together complementary skills to answer major scientific questions from each KQ.
Flagship project KQ1-CDI1: “Towards therapeutic antibodies with pH-switchable properties”